Understanding Chloromethyl Methyl Ether: Synthesis and Characterization
Chloromethyl methyl ether (CMME) is a versatile and reactive organic compound that plays a significant role in synthetic chemistry, particularly as an alkylating agent. This article delves into the synthesis and characterization of CMME, along with its industrial relevance, safety concerns, and future potential in the field of chemical research and applications.
Introduction to Chloromethyl Methyl Ether
Overview of Chloromethyl Methyl Ether (CMME)
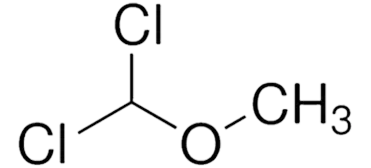
Chloromethyl methyl ether (CMME) is a highly reactive compound with the chemical formula CH₃OCH₂Cl. Known for its powerful alkylating properties, it is often used in organic synthesis, particularly in the production of polymers and resins. CMME acts as a key intermediate in the synthesis of numerous organic molecules, particularly those used in industrial manufacturing processes.
Due to its reactivity, CMME is typically handled with caution, as it poses several risks to both human health and the environment. It is classified as a hazardous substance, meaning that its synthesis and use require specialized knowledge and equipment to ensure safe handling. The chemical’s structure, featuring both an ether group (-O-) and a chloromethyl group (-CH₂Cl), is responsible for its wide range of chemical applications.
Significance in Chemical Industries
CMME’s widespread use in chemical industries cannot be understated. It is a crucial reagent in the manufacture of adhesives, coatings, and plastics. Furthermore, it plays an important role in pharmaceutical synthesis, where it contributes to the production of active ingredients through alkylation. Given its industrial significance, understanding both its synthesis and the techniques used to characterize it is essential for chemists working in various fields.
Synthesis of Chloromethyl Methyl Ether
Basic Chemistry of CMME Formation
The synthesis of chloromethyl methyl ether generally involves a reaction between methanol (CH₃OH) and formaldehyde (CH₂O) in the presence of hydrogen chloride (HCl). The chloromethylation process introduces a chloromethyl group (-CH₂Cl) to the methyl ether, resulting in the formation of CMME. The reaction can be expressed as:
CH3OH+CH2O+HCl→CH3OCH2Cl+H2OCH₃OH + CH₂O + HCl → CH₃OCH₂Cl + H₂O
This simple yet effective synthetic route is widely employed in both laboratory and industrial settings. However, due to the reactivity of the reagents involved, especially formaldehyde and HCl, the synthesis process must be carefully controlled to prevent unwanted side reactions and ensure the safe production of the target compound.
Raw Materials for CMME Synthesis
The primary raw materials for synthesizing CMME are methanol, formaldehyde, and hydrochloric acid. Methanol, a simple alcohol, acts as the starting material that forms the ether bond, while formaldehyde provides the carbon backbone needed for the chloromethyl group. Hydrochloric acid serves as both a reactant and a catalyst, facilitating the formation of the chloromethyl group through nucleophilic substitution.
High-purity reagents are typically required for CMME synthesis, as impurities can lead to the formation of by-products, reducing the yield and purity of the final product.
Industrial and Laboratory Synthesis Methods
In industrial settings, CMME is synthesized on a large scale under controlled conditions using specialized equipment. The process often involves continuous flow reactors, which allow for precise control over the reaction parameters, such as temperature and pressure. This ensures a consistent product with minimal impurities.
Laboratory synthesis, on the other hand, typically occurs in smaller quantities using batch reactors. While the fundamental chemistry remains the same, laboratory-scale synthesis often involves additional purification steps, such as distillation or crystallization, to isolate the pure CMME from the reaction mixture.
Mechanism of Chloromethyl Methyl Ether Synthesis
Reaction Pathways
The reaction mechanism of CMME synthesis is primarily based on a nucleophilic substitution reaction. In this process, the hydroxyl group (-OH) of methanol is replaced by a chloromethyl group (-CH₂Cl). Formaldehyde plays a crucial role in the reaction, as it provides the necessary carbon atom that links the methyl ether group with the chlorine atom.
The initial step involves the formation of a hemiacetal intermediate when methanol reacts with formaldehyde. This intermediate then undergoes protonation by hydrochloric acid, which activates it for nucleophilic attack by chloride ions. The chloride ion then displaces the hydroxyl group, resulting in the formation of CMME.
Catalysts and Reaction Conditions
The synthesis of CMME typically requires acidic conditions to proceed efficiently. Hydrochloric acid serves as both a reactant and a catalyst, facilitating the chloromethylation reaction. Temperature is another critical factor in the reaction, with most syntheses being carried out at moderately elevated temperatures (40-60°C). This temperature range provides sufficient energy for the reaction to proceed while minimizing the risk of thermal decomposition of the product.
In some cases, catalysts such as Lewis acids may be added to improve reaction efficiency and yield. However, these catalysts can introduce additional complexity to the process, requiring careful optimization of reaction conditions.
Characterization of Chloromethyl Methyl Ether
Physical and Chemical Properties
Chloromethyl methyl ether is a colorless liquid with a sharp, pungent odor. It is highly volatile and exhibits a boiling point of around 59-60°C. CMME is miscible with most organic solvents but exhibits limited solubility in water. Its vapor is highly flammable, and it poses significant health risks if inhaled or absorbed through the skin.
Chemically, CMME is reactive due to the presence of the chloromethyl group, which readily participates in nucleophilic substitution reactions. This reactivity makes it valuable in synthetic organic chemistry, where it can be used to introduce alkyl groups into a wide range of molecules.
5-bromo-2-4-dichloropyrimidine is a significant heterocyclic compound used in various pharmaceutical applications.
Analytical Techniques for CMME Identification
Characterization of chloromethyl methyl ether involves various analytical techniques to confirm its structure and purity. The most common techniques used for CMME analysis include:
- Gas Chromatography (GC): GC is frequently employed to assess the purity of CMME by separating and quantifying its components.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR provides detailed information about the molecular structure of CMME, particularly the arrangement of its atoms.
- Infrared (IR) Spectroscopy: IR spectroscopy is used to identify functional groups within the CMME molecule, particularly the characteristic C-O and C-Cl stretches.
- Mass Spectrometry (MS): MS can be used to determine the molecular weight and fragmentation pattern of CMME, further confirming its identity.
These techniques are critical for ensuring the quality and consistency of CMME in both research and industrial applications.
Hazards and Safety Concerns
Toxicological Profile
Chloromethyl methyl ether is classified as a highly toxic and carcinogenic substance. Exposure to CMME, even in small quantities, can lead to severe health effects, including respiratory irritation, skin burns, and long-term damage to internal organs. Chronic exposure is associated with an increased risk of cancer, particularly lung cancer.
Because of these risks, strict safety protocols must be followed when working with CMME. Personal protective equipment (PPE), including gloves, goggles, and respirators, is essential for minimizing exposure. Bromoacetonitrile is a versatile organic compound commonly used as an intermediate in the synthesis of pharmaceuticals and agrochemicals.
Handling and Storage Requirements
CMME should be stored in tightly sealed containers in a well-ventilated area away from heat sources and incompatible materials, such as strong oxidizers. Proper labeling and safety data sheets (SDS) must be available to all personnel handling the chemical.

